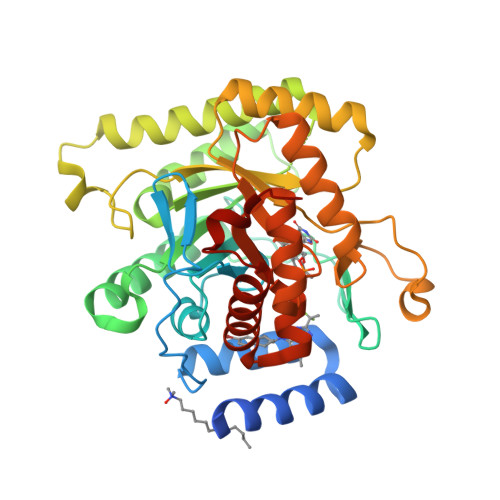
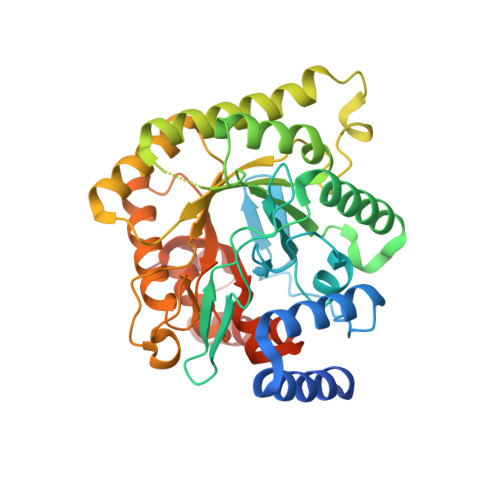
Tetrahydro-2-naphthyl and 2-Indanyl Triazolopyrimidines Targeting Plasmodium falciparum Dihydroorotate Dehydrogenase Display Potent and Selective Antimalarial Activity.
Kokkonda, S., Deng, X., White, K.L., Coteron, J.M., Marco, M., de Las Heras, L., White, J., El Mazouni, F., Tomchick, D.R., Manjalanagara, K., Rudra, K.R., Chen, G., Morizzi, J., Ryan, E., Kaminsky, W., Leroy, D., Martinez-Martinez, M.S., Jimenez-Diaz, M.B., Bazaga, S.F., Angulo-Barturen, I., Waterson, D., Burrows, J.N., Matthews, D., Charman, S.A., Phillips, M.A., Rathod, P.K.(2016) J Med Chem 59: 5416-5431
- PubMed: 27127993
- DOI: https://doi.org/10.1021/acs.jmedchem.6b00275
- Primary Citation of Related Structures:
5FI8 - PubMed Abstract:
Malaria persists as one of the most devastating global infectious diseases. The pyrimidine biosynthetic enzyme dihydroorotate dehydrogenase (DHODH) has been identified as a new malaria drug target, and a triazolopyrimidine-based DHODH inhibitor 1 (DSM265) is in clinical development. We sought to identify compounds with higher potency against Plasmodium DHODH while showing greater selectivity toward animal DHODHs. Herein we describe a series of novel triazolopyrimidines wherein the p-SF5-aniline was replaced with substituted 1,2,3,4-tetrahydro-2-naphthyl or 2-indanyl amines. These compounds showed strong species selectivity, and several highly potent tetrahydro-2-naphthyl derivatives were identified. Compounds with halogen substitutions displayed sustained plasma levels after oral dosing in rodents leading to efficacy in the P. falciparum SCID mouse malaria model. These data suggest that tetrahydro-2-naphthyl derivatives have the potential to be efficacious for the treatment of malaria, but due to higher metabolic clearance than 1, they most likely would need to be part of a multidose regimen.
Organizational Affiliation:
Departments of Chemistry and Global Health, University of Washington , Seattle, Washington 98195, United States.